SPR-315A SPR-315B SPR-318A SPR-315C SPR-318B SPR-318C +2
HO-1 Protein
Stressmarq Biosciences
DESCRIPTION
Rat Recombinant HO-1 Partial Protein
DETAILS
- Nature: Recombinant
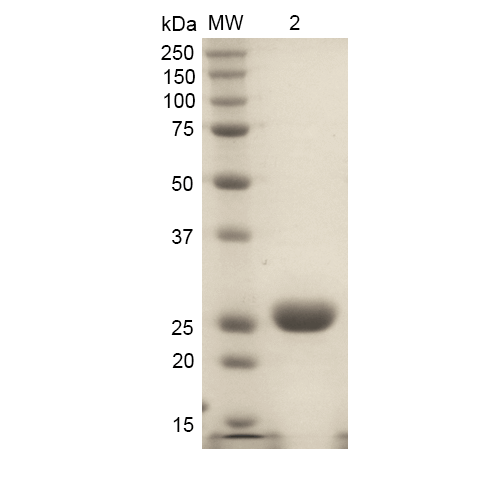
- Purity: >90%
- Target: HO-1
- Category: Protein
- Conjugate: His tag
- References: 1. Froh M. et al. (2007) World J. Gastroentereol 13(25): 3478-86. 2. Elbirt K.K. and Bonkovsky H.L. (1999) Proc Assoc Am Physicians 111(5): 348-47. 3. Maines M.D., Trakshel G.M., and Kutty R.K. (1986) J Biol Chem 261: 411–419. 4. Brydun A., et al. (2007) Hypertens Res 30(4): 341-8. 5. Poss K.D. and Tonegawa S. (1997). Proc Natl Acad Sci U S A. 94: 10925–10930. 6. Yet S.F., et al. (2003) FASEB J. 17: 1759–1761. 7. Yachie A., et al. (1999) J Clin Invest. 103: 129–135.
- Applications: WB | SDS-PAGE
- Field of Use: Not for use in humans. Not for use in diagnostics or therapeutics. For research use only.
- Protein Size: ~32 kDa
- Purification: Affinity Purified
- Concentration: Lot/batch specific. See included datasheet.
- Protein Length: Partial
- Research Areas: Cancer | Oxidative Stress
- Storage Buffer: 50mM Tris/HCl pH7.5, 5mM Bme, 0.15NaCl, 10% glycerol
- Alternative Names: Heme oxygenase 1 Protein, Hemox Protein, HMOX1 Protein, HO1 Protein, HO 1 Protein, HSP32 Protein
- Cite This Product: Rat Recombinant HO-1 Protein (StressMarq Biosciences, Canada, Cat # SPR-315A)
- Expression System: E. coli
- Species Full Name: Rat
- Amino Acid Sequence: MERPQLDSMSQDLSEALKEATKEVHIRAENSEFMRNFQKGQVSREGFKLVMASLYHIYTALEEEIERNKQNPVYAPLYFPEELHRRAALEQDMAFWYGPHWQEAIPYTPATQHYVKRLHEVGGTHPELLVAHAYTRYLGDLSGGQVLKKIAQKAMALPSSGEGLAFFTFPSIDNPTKFKQLYRARMNTLEMTPEVKHRVTEEAKTAFLLNIELFEELQALLTEEHKDQSPSQTEFLRQRPASLVQDTTSAETPRGKSQIST
- Storage Temperature: -20ºC
- Shipping Temperature: Blue Ice or 4ºC
- Cellular Localization: Microsome | Endoplasmic Reticulum
- Scientific Background: Heme-oxygenase is a ubiquitous enzyme that catalyzes the initial and rate-limiting steps in heme catabolism yielding equimolar amounts of biliverdin, iron and carbon monoxide. Biliverdin is subsequently converted to bilirubin and the free iron is sequestered to ferritin (1). These products have important physiological effects as carbon monoxide is a potent vasodilator; biliverdin and bilirubin are potent antioxidants; and the free iron increases oxidative stress and regulates the expression of many mRNAs (2).There are three isoforms of heme-oxygenase, HO-1, HO-2 and HO-3; however HO-1 and HO-2 are the major isoforms as they both have been identified in mammals (3). HO-1, also known as heat shock protein 32, is an inducible isoform activated by most oxidative stress inducers, cytokines, inflammatory agents and heat shock. HO-2 is a constitutive isoform which is expressed under homeostatic conditions. HO-1 is also considered to be a cytoprotective factor in that free heme is highly reactive and cytotoxic, and secondly, carbon monoxide is a mediator inhibiting the inflammatory process and bilirubin is a scavenger for reactive oxygen, both of which are the end products of heme catalyzation (4). It has also been shown that HO-1 deficiency may cause reduced stress defense, a pro-inflammatory tendency (5), susceptibility to atherosclerotic lesion formation (6), endothelial cell injury, and growth retardation (7). Up-regulation of HO-1 is therefore said to be one of the major defense mechanisms of oxidative stress (4).
- Certificate of Analysis: This product has been certified >90% pure using SDSPAGE analysis.