78668
HRE Luciferase Reporter Lentivirus
BPS Bioscience
DESCRIPTION
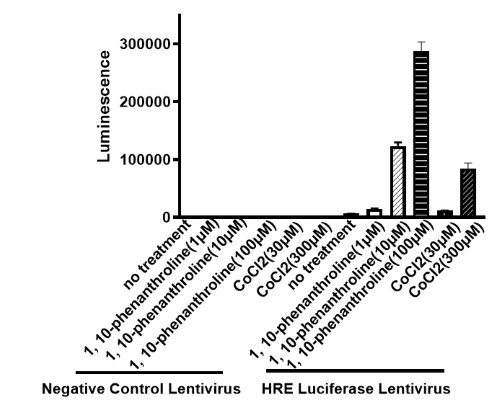
The Hypoxia Response Element (HRE) Luciferase Reporter Lentiviruses are replication incompetent, HIV-based, VSV-G pseudotyped lentiviral particles that are ready to transduce most types of mammalian cells, including primary and non-dividing cells. The particles contain a firefly luciferase gene driven by four copies of a hypoxia response elements (HRE) located upstream of the minimal TATA promoter (Figure 1) and an antibiotic selection gene (puromycin) for the selection of stable clones. After transduction, the induction of hypoxia in the target cells can be monitored by measuring the luciferase activity.
DETAILS
- Notes: To generate a HRE luciferase reporter stable cell line, remove the growth medium 48 hours after transduction and replace it with fresh growth medium containing the appropriate amount of puromycin for antibiotic selection of transduced cells. To determine the concentration of puromycin needed for your cell line, perform a kill curve (for more information, visit our Resources page, frequently asked questions: what is a kill curve?). The following Lentivirus Reporter Controls are available from BPS Bioscience to meet your experimental needs: Negative Control Luciferase Lentivirus (BPS Bioscience #79578): Ready-to-transduce lentiviral particles expressing firefly luciferase under the control of a minimal promoter. The negative control is important to establish the specificity of any treatments and to determine the background reporter activity. Renilla Luciferase Lentivirus (BPS Bioscience #79565): Ready-to-transduce lentiviral particles expressing Renilla luciferase under the CMV promoter. The Renilla Luciferase lentivirus can serve as an internal control to overcome sample-to-sample variability when performing dual-luciferase reporter assays. Firefly Luciferase Lentivirus (BPS Bioscience #79692-G, #79692-H, #79692-P): Ready-to-transduce lentiviral particles expressing firefly luciferase under the CMV promoter. It serves as a positive control for transduction optimization studies. Biosafety: The lentiviruses are produced with SIN (self-inactivation) lentivector which ensures self-inactivation of the lentiviral construct after transduction and integration into the genomic DNA of the target cells. None of the HIV genes (gag, pol, rev) will be expressed in the transduced cells, as they are expressed from packaging plasmids lacking the packing signal and are not present in the lentivirus particle. Although the pseudotyped lentiviruses are replication-incompetent, they require the use of a Biosafety Level 2 facility. BPS Bioscience recommends following all local federal, state, and institutional regulations and using all appropriate safety precautions. Troubleshooting Guide: Visit bpsbioscience.com/lentivirus-faq for detailed troubleshooting instructions. For all further questions, please email support@bpsbioscience.com.
- Shiptemp: -80°C (dry ice)
- Warnings: Avoid freeze/thaw cycles.
- Category: Immunotherapy/Lentivirus
- Background: Hypoxia occurs in solid tumors as a result of poor vascularization within the core of the tumor. It is a driver of tumor progression and resistance to therapy through adaptive responses. Hypoxia response elements (HREs) are transcription factor binding sites within the promoters of various genes regulated by hypoxia-inducible factors (HIFs). As oxygen becomes rate limiting, HIFs form heterodimers that recognize cognate HREs, thus activating the transcription of genes involved in cell proliferation, metastasis, and angiogenesis. HIF activation in many types of cancer correlates with poor outcomes.
- Description: The Hypoxia Response Element (HRE) Luciferase Reporter Lentiviruses are replication incompetent, HIV-based, VSV-G pseudotyped lentiviral particles that are ready to transduce most types of mammalian cells, including primary and non-dividing cells. The particles contain a firefly luciferase gene driven by four copies of a hypoxia response elements (HRE) located upstream of the minimal TATA promoter (Figure 1) and an antibiotic selection gene (puromycin) for the selection of stable clones. After transduction, the induction of hypoxia in the target cells can be monitored by measuring the luciferase activity.
- Formulation: The lentivirus particles were produced from HEK293T cells. They are supplied in cell culture medium containing 90% DMEM + 10% FBS.
- Supplied As: Two vials (500 µl x 2) of lentivirus at a titer >107 TU/ml. The titer will vary with each lot; the exact value will be provided with each shipment.
- Unspsc Code: 41106621
- Unspsc Name: Virus mediated expression vectors or kits
- Applications: Screen for activators or inhibitors of hypoxia-related signaling pathways Generate an HRE luciferase reporter stable cell line (puromycin resistant) following puromycin selection and limiting dilution
- Product Type: Lentivirus
- Biosafety Level: BSL-2
- Related Products: 60513, 78666, 78672
- Storage Stability: Lentiviruses are shipped with dry ice. For long-term storage, it is recommended to store the lentiviruses at -80°C.
- Scientific Category: Immunotherapy