SPR-435B SPR-435C SPR-435E
Scientist.com Supplier
Superoxide Dismutase Monomers
Stressmarq Biosciences
Image
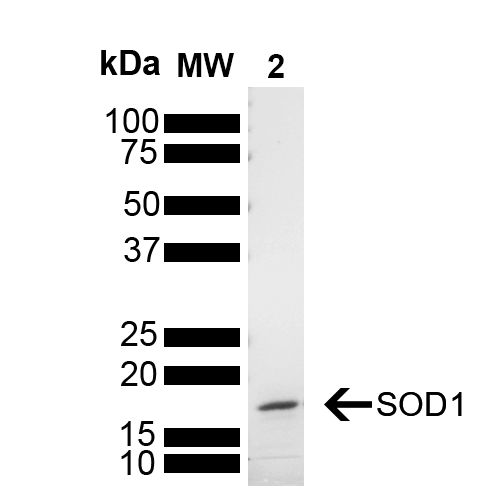
Image
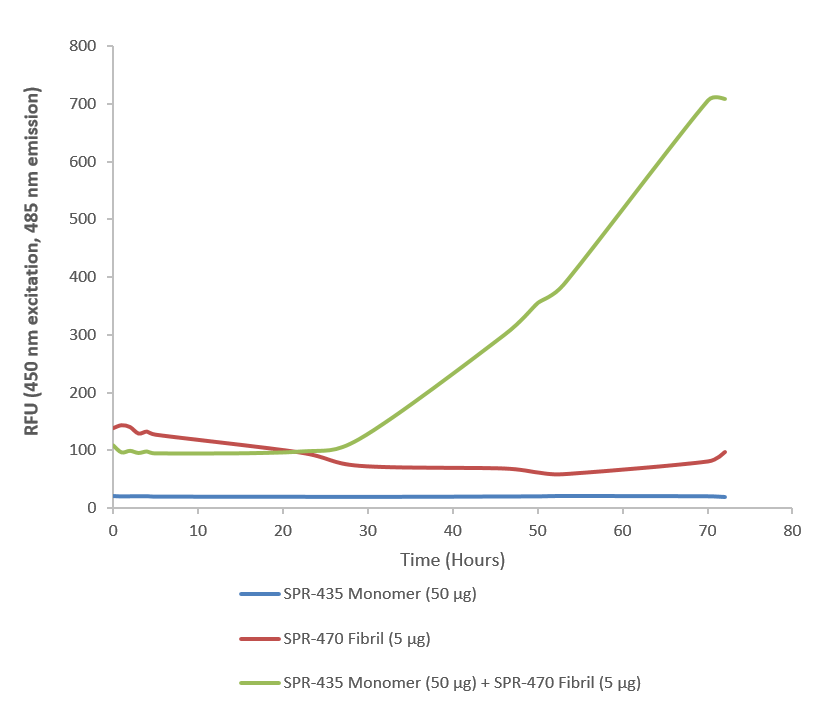
DESCRIPTION
Human Recombinant Superoxide Dismutase Protein Monomers
DETAILS
- Nature: Recombinant
- Purity: >95%
- Target: Superoxide Dismutase (SOD)
- Category: Protein
- Conjugate: No Tag
- References: 1. Adachi T., et al. (1992). Clin. Chim. Acta. 212: 89-102. 2. Barrister J.V., et al. (1987). Crit. Rev. Biochem. 22:111-180. 3. Furukawa Y., O'Halloran T. (2006). Antioxidants & Redo Signaling. Vol 8, No 5,6. 4. Gao B., et al. (2003). Am J Physiol Lung Cell Mol Physiol 284: L917-L925. 5. Hassan H.M. (1988). Free Radical Biol. Med. 5: 377-385. 6. Kurobe N., et al. (1990) Biomedical Research. 11: 187-194 7. Wispe J.R., et al. (1989) BBA. 994: 30-36. 8. Xiao-Hong Liu., et al. (1993) Brain Research. 625: 29-37. 9. Furukawa Y., et al. (2013) FEBS 587(16): 2500-2505.
- Applications: WB | SDS-PAGE | In vivo assay | In vitro assay
- Field of Use: Not for use in humans. Not for use in diagnostics or therapeutics. For research use only.
- Protein Size: 15.936 kDa
- Purification: Ion-exchange Purified
- Concentration: Lot/batch specific. See included datasheet.
- Protein Length: Full length
- Research Areas: Cancer | Oxidative Stress | Cell Signaling | Protein Trafficking | Chaperone Proteins | Neuroscience | Neurodegeneration | ALS Disease
- Storage Buffer: PB pH 7.4
- Alternative Names: Superoxide dismutase1 Protein Monomer, ALS1 Protein Monomer, SOD1 Protein Monomer, IPOA Protein Monomer
- Cite This Product: Human Recombinant SOD Protein (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-435B)
- Expression System: E. coli
- Species Full Name: Human
- Storage Temperature: -80ºC
- Shipping Temperature: Dry Ice. Shipping note: Product will be shipped separately from other products purchased in the same order.
- Cellular Localization: Nucleus | Mitochondrion | Cytoplasm
- Scientific Background: Superoxide dismutase (SOD) is an endogenously produced intracellular enzyme present in almost every cell in the body (3). It works by catalyzing the dismutation of the superoxide radical O2ˉ to O2 and H2O2, which are then metabolized to H2O and O2 by catalase and glutathione peroxidase (2,5). In general, SODs play a major role in antioxidant defense mechanisms (4). There are two main types of SOD in mammalian cells. One form (SOD1) contains Cu and Zn ions as a homodimer and exists in the cytoplasm. The two subunits of 16 kDa each are linked by two cysteines forming an intra-subunit disulphide bridge (3). The second form (SOD2) is a manganese containing enzyme and resides in the mitochondrial matrix. It is a homotetramer of 80 kDa. The third form (SOD3 or EC-SOD) is like SOD1 in that it contains Cu and Zn ions, however it is distinct in that it is a homotetramer, with a mass of 30 kDA and it exists only in the extra-cellular space (7). SOD3 can also be distinguished by its heparin-binding capacity (1). Studies have shown that in vitro, Cu-Zn SOD (SOD1) fibrils are transduced into cells and function as seeds to trigger the aggregation of endogenously expressed SOD1 (9).
- Certificate of Analysis: Certified >95% pure using SDS-PAGE analysis.
Equivalent Items
| ... Loading